INDICATION
RYTELO™ (imetelstat) is indicated for the treatment of adult patients with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring 4 or more red blood cell units over 8 weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESA). See more
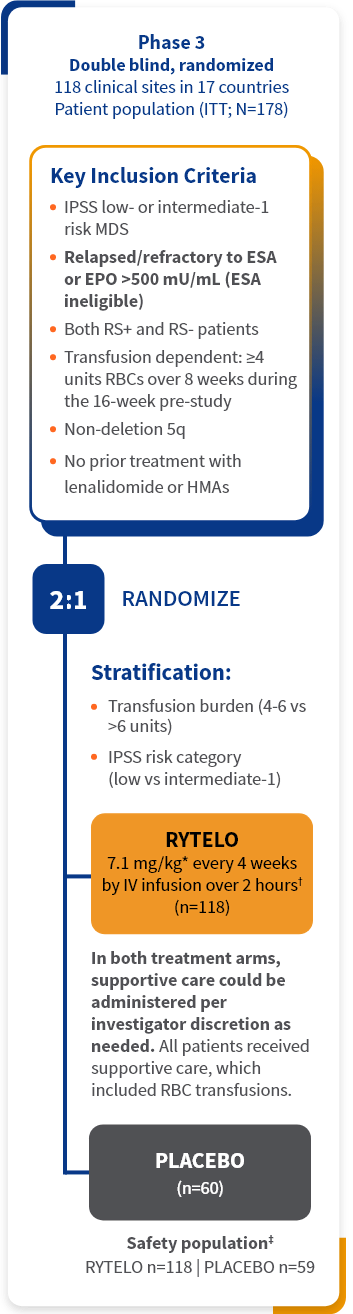
RYTELO was studied in the IMerge phase 3 clinical trial1
The IMerge phase 3 clinical trial assessed the safety and efficacy of RYTELO (n=118) vs placebo (n=60) in LR-MDS patients with transfusion-dependent anemia1-3
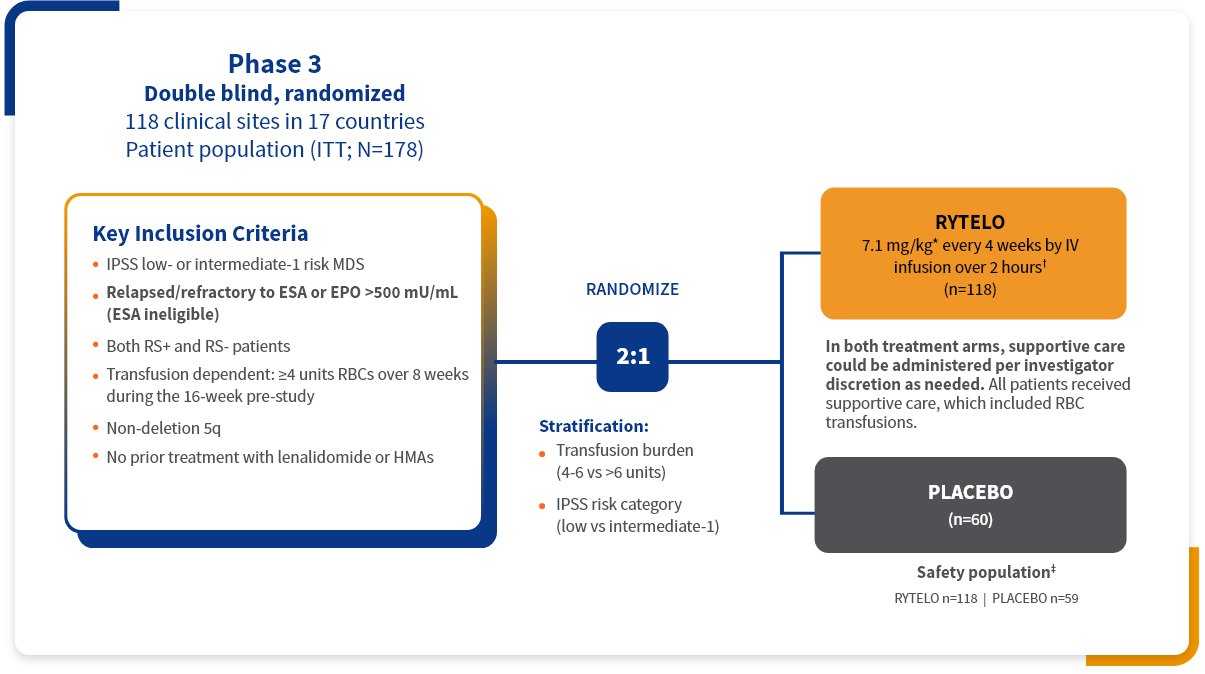
Primary endpoint:
- ≥8 consecutive weeks of RBC-TI§
Key secondary endpoints:
- ≥24 consecutive weeks of RBC-TI||
- Duration of response in patients who achieved ≥8-week RBC-TI
- Rate of hematologic improvement–erythroid, measured by Hgb increase or RBC unit reduction (HI-E IWG 2006)
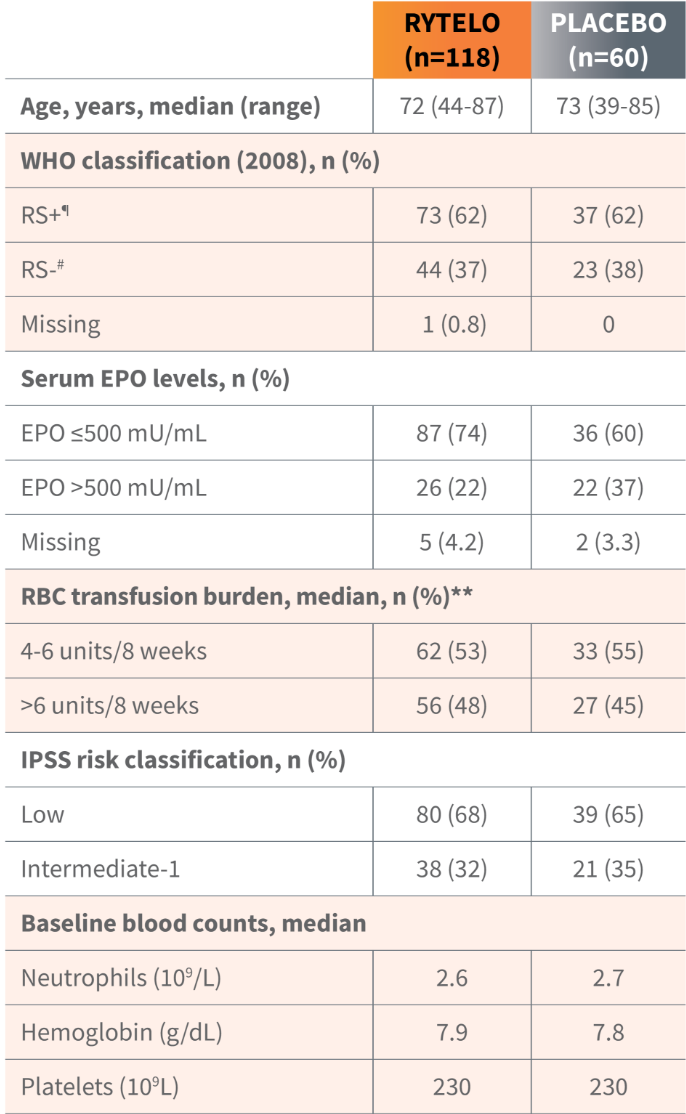
Key baseline patient demographics/disease characteristics1,2
Patients in the IMerge trial included those with high transfusion burden, those who were ESA ineligible (EPO >500 mU/mL), or ESA relapsed/refractory and transfusion-dependent anemia requiring ≥4 RBC units/8 weeks, as well as RS-positive and RS-negative individuals.3

EPO, erythropoietin; ESA, erythropoiesis-stimulating agent; Hgb, hemoglobin; HMA, hypomethylating agent; IPSS, International Prognostic Scoring System; ITT, intent to treat; IV, intravenous; LR-MDS, lower-risk myelodysplastic syndromes; RBC, red blood cell; RBC-TI, red blood cell transfusion independence; RS, ring sideroblasts; WHO, World Health Organization.
References: 1. RYTELO. Prescribing information. Geron Corp.; 2024. 2. Data on file. Geron Corporation. Foster City, CA. 3. Platzbecker U, Santini V, Fenaux P, et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): a multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024;403(10423):249-260.
INDICATION
RYTELO™ (imetelstat) is indicated for the treatment of adult patients with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring 4 or more red blood cell units over 8 weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESA).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Thrombocytopenia
RYTELO can cause thrombocytopenia based on laboratory values. In the clinical trial, new or worsening Grade 3 or 4 decreased platelets occurred in 65% of patients with MDS treated with RYTELO.
Monitor patients with thrombocytopenia for bleeding. Monitor complete blood cell counts prior to initiation of RYTELO, weekly for the first two cycles, prior to each cycle thereafter, and as clinically indicated. Administer platelet transfusions as appropriate. Delay the next cycle and resume at the same or reduced dose, or discontinue as recommended.
Neutropenia
RYTELO can cause neutropenia based on laboratory values. In the clinical trial, new or worsening Grade 3 or 4 decreased neutrophils occurred in 72% of patients with MDS treated with RYTELO.
Monitor patients with Grade 3 or 4 neutropenia for infections, including sepsis. Monitor complete blood cell counts prior to initiation of RYTELO, weekly for the first two cycles, prior to each cycle thereafter, and as clinically indicated. Administer growth factors and anti-infective therapies for treatment or prophylaxis as appropriate. Delay the next cycle and resume at the same or reduced dose, or discontinue as recommended.
Infusion-Related Reactions
RYTELO can cause infusion-related reactions. In the clinical trial, infusion-related reactions occurred in 8% of patients with MDS treated with RYTELO; Grade 3 or 4 infusion-related reactions occurred in 1.7%, including hypertensive crisis (0.8%). The most common infusion-related reaction was headache (4.2%). Infusion-related reactions usually occur during or shortly after the end of the infusion.
Premedicate patients at least 30 minutes prior to infusion with diphenhydramine and hydrocortisone as recommended and monitor patients for one hour following the infusion as recommended. Manage symptoms of infusion-related reactions with supportive care and infusion interruptions, decrease infusion rate, or permanently discontinue as recommended.
Embryo-Fetal Toxicity
RYTELO can cause embryo-fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RYTELO and for 1 week after the last dose.
ADVERSE REACTIONS
Serious adverse reactions occurred in 32% of patients who received RYTELO. Serious adverse reactions in >2% of patients included sepsis (4.2%), fracture (3.4%), cardiac failure (2.5%), and hemorrhage (2.5%). Fatal adverse reactions occurred in 0.8% of patients who received RYTELO, including sepsis (0.8%).
Most common adverse reactions (≥10% with a difference between arms of >5% compared to placebo), including laboratory abnormalities, were decreased platelets, decreased white blood cells, decreased neutrophils, increased AST, increased alkaline phosphatase, increased ALT, fatigue, prolonged partial thromboplastin time, arthralgia/myalgia, COVID-19 infections, and headache.
Please see full Prescribing Information, including Medication Guide.
You are encouraged to report adverse events related to Geron products by calling 1-855-437-6664 (1-855-GERON-MI) (US only). If you prefer, you may contact the US Food and Drug Administration (FDA) directly. Visit www.fda.gov/MedWatch or call 1-800-FDA-1088.
